|
|
|
Plants need water, air, light, suitable temperature, and nutrients to grow. Plants get carbon, hydrogen and oxygen from air and water. Forty years ago, thirteen elements were listed as essential nutrients which plants get from soil. Recent testing has now identified 42 essential nutrients but not all are needed by all plants.
The six that the plants need the most of are called Macronutrients. They are Nitrogen (N), Phosphorus (P), Potassium (K), Calcium (C), Magnesium (Mg) and Sulfur (S). The other nutrients, which are needed only in trace amounts, are called Micronutrients. They include Arsenic (As), Boron (B), Chlorine (Cl), Cobalt (Co), Copper (Cu), Iron (Fe), Manganese (Mn), Molybdenum (Mo), Nickel (Ni), Selenium (Se), Silicon (Si), Sodium (Na), Vanadium (V) and Zinc (Zn).
These
 nutrients are essential for plant growth. Plants will grow normally until they run short of
one nutrient. Then growth is limited by the availability of that nutrient. Occasionally two or more
nutrients will run short at the same time. If the nutrients are deficient, or too abundant, then
plants will be discolored or deformed. Deficiency symptoms might indicate which nutrient is needed,
but the symptoms vary from one kind of plant to another. Some nutrient deficiencies cause patterns
in leaves that follow the veins. It might be green veins and yellow in between or yellow veins and
green in between. Some deficiencies cause purple or brown areas. Disease symptoms do not follow the
veins.
nutrients are essential for plant growth. Plants will grow normally until they run short of
one nutrient. Then growth is limited by the availability of that nutrient. Occasionally two or more
nutrients will run short at the same time. If the nutrients are deficient, or too abundant, then
plants will be discolored or deformed. Deficiency symptoms might indicate which nutrient is needed,
but the symptoms vary from one kind of plant to another. Some nutrient deficiencies cause patterns
in leaves that follow the veins. It might be green veins and yellow in between or yellow veins and
green in between. Some deficiencies cause purple or brown areas. Disease symptoms do not follow the
veins.
It is much better to supply additional nutrients before deficiency symptoms appear. A soil test or leaf analysis will tell which nutrients are low before growth is affected. Soil pH can be checked with a pH meter, or soil laboratories will test soils for nutrients as well as pH and organic matter. A local soil testing lab, A & L Western Laboratories, is in Sherwood, OR. They also do tissue testing to see what the nutrient levels are inside the plant because sometimes nutrients in the soil are not being absorbed into the plants. They can also provide a chart telling what nutrients and how much lime to add to soil depending on what is grown on it.
Nitrogen (N) stimulates leaf and stem growth. Nitrogen deficiency causes reduced growth and pale yellowish green leaves. The older leaves turn yellowish first since the nitrogen is readily moved from the old leaves to the new growth. If the soil is cold and wet, nitrogen in the soil is not as available to the plants. Excess nitrogen might cause potassium deficiency.
Phosphorus (P) is important in the germination and growth of seeds, the production of flowers and fruit, and the growth of roots. Phosphorus deficiency causes reduced growth and small leaves that drop early, starting with the oldest leaves. Leaf color is a dull, bluish green that becomes purplish or bronzy. Leaf edges often turn scorched brown. Excess phosphorus might cause potassium deficiency.
Potassium (K) promotes general vigor, disease resistance and sturdy growth. Potassium deficiency causes stunted growth with leaves close together. Starting with the older leaves, the leaf tips and edges turn scorched brown and leaf edges roll.
Calcium (Ca) is a major ingredient in cell walls and is important for root growth, especially root tips. Calcium deficiency causes poorly developed roots with weak tips. Leaves are distorted with hooked tips and curled margins that often turn brown.
Magnesium (Mg) is vital to chlorophyll production and is important in most enzyme reactions. Magnesium deficiency causes different symptoms in different plants, but commonly includes leaf yellowing with brilliant tints. Leaves might suddenly drop off without withering. Symptoms show first on older leaves.
An excessive amount of Potassium (K), Calcium (Ca) or Magnesium (Mg) can interfere with the absorption of the other two. A general rule is to provide Potassium, Calcium and Magnesium in a 4:3:1 ratio. Excessive Sodium (Na) from fertilizer or water, especially softened water, can also interfere with Potassium, Calcium and Magnesium uptake. They are all positively charged ions so they are absorbed by the same path.
Sulfur (S) is an ingredient in proteins and is necessary for chlorophyll formation. Sulfur deficiency causes slow growth with small round leaves that roll upward and are stiff and brittle. Leaves drop off and tip buds die.
Boron (B) is necessary for the movement of sugars, for reproduction, and for water intake by cells. It also tends to keep calcium in a soluble form. Boron deficiency causes distorted and dead growing tips, hollow stems and deformed fruit. Leaves are often scorched and curled and sometimes mottled, discolored and thickened. Young leaves are affected first. Excess boron might cause scorched leaf edges similar to potassium or magnesium deficiencies.
Chlorine, Cl, is important for osmosis and photosynthesis. Chlorine deficiencies might cause stubby roots and wilting. Excess chlorine might cause leaf edges to scorch similar to potassium deficiency. Chlorine in treated water might be two to four times higher than plants need.
Cobalt (Co) is beneficial for some plants but it is essential for nitrogen fixing bacteria associated with legumes.
Copper (Cu) is necessary for the production of proteins and is important for reproduction. Copper deficiency causes bluish green leaves which might wither or fail to unfold. Younger leaf tips might be yellow at the edge. Growing tips might form rosettes. Excess copper might cause iron deficiency.
Iron (Fe) is necessary for chlorophyll formation and for oxygen transfer. Iron deficiency causes leaf yellowing while leaf veins stay green. Younger leaves are affected first because iron does not move from old leaves to new leaves. Excess lime might cause iron deficiency.
Manganese (Mn) is a catalyst for many enzymes and is important for chlorophyll formation. Manganese deficiency causes different symptoms in different plants, but commonly causes leaves to turn yellow while veins stay green. White or gray specks may appear on leaves. Older leaves are affected first. Excess manganese might cause iron deficiency and might cause symptoms similar to manganese deficiency.
Molybdenum (Mo) is essential to nitrate enzymes and for the formation of root nodules in beans and peas. Molybdenum deficiency causes yellow mottling and dead spots on the leaves. In some plants, the growing tips are distorted or killed.
Nickel (Ni) activates certain enzymes and can be a substitute for iron and zinc in some enzymes.
Selenium (Se) can increase plant growth and increase resistance to diseases. Deficiency symptoms include stunted growth, leaf chlorosis and poor seed germination.
Silicon (Si) makes cell walls tougher so they are more resistant to fungal diseases and to piercing and sucking insects.
Sodium (Na) can increase leaf surface and the number of stomata and also replace potassium in some processes.
Vanadium (V) is essential for some plants and can substitute for molybdenum in some processes.
Zinc (Zn) is necessary for the production of proteins and affects plant size and maturity. Zinc deficiency causes leaf yellowing between the veins, usually with purple or dead spots starting with the older leaves. Leaves are close together, small and deformed. Fruiting is reduced. Excess zinc might cause iron deficiency.
Some
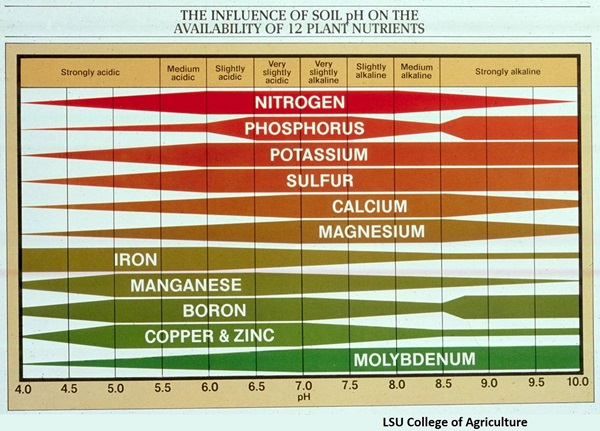 nutrients might be plentiful in the soil but deficient in plants because they are bound up in
chemical compounds that make them unavailable to plants. Soil pH can make a big difference in
nutrient availability. Soil pH is a measure of hydrogen ion concentration. It is tested either with a
chemical pH test, or by a pH meter. 7.0 is neutral. 4.0 is very acid. 10.0 is very alkaline. High
rainfall and high organic matter produces acid soil. Low rainfall and high lime or sodium produces
alkaline soils. Soils in western Oregon naturally have a pH between 4.5 and 5.5. Soils in eastern
Oregon commonly have a pH above 8.
nutrients might be plentiful in the soil but deficient in plants because they are bound up in
chemical compounds that make them unavailable to plants. Soil pH can make a big difference in
nutrient availability. Soil pH is a measure of hydrogen ion concentration. It is tested either with a
chemical pH test, or by a pH meter. 7.0 is neutral. 4.0 is very acid. 10.0 is very alkaline. High
rainfall and high organic matter produces acid soil. Low rainfall and high lime or sodium produces
alkaline soils. Soils in western Oregon naturally have a pH between 4.5 and 5.5. Soils in eastern
Oregon commonly have a pH above 8.
The majority of plant nutrients are most available at slightly acid to neutral, a pH of 6-7. The availability of phosphorus drops off rapidly as pH drops below 6.5, but iron is more available as soil is more acid. Acid loving plants, such as camellias, azaleas and rhododendrons, need to grow in an acidic soil in order to get enough iron. Micronutrients are also more available when soil is more acid. Most plants get the micronutrients they need when soil pH is 5.8 to 6.2.
Soil can be made less acid by adding lime (calcium carbonate), or more acid by adding sulfur or aluminum sulfate. Lime will raise the soil pH in a few weeks or several months depending on how finely ground the lime particles are. Sulfur takes about a year to lower soil pH since bacteria are required to complete the process.
Nutrient availability is greatly enhanced if mycorrhizae are present in the soil. Mycorrhizae are
beneficial fungi, the white netting growing out of the brown roots in the picture below. The network
of mycorrhizae can absorb four or five times as much water and nutrients as is absorbed directly
through roots. They seem to be much better than roots at extracting phosphorus from acid soil.

Most plants in most soils will grow better if additional nutrients are provided by fertilizing. Soil laboratories will test soils for nutrients as well as pH and organic matter. A local soil testing lab, A&L Western Laboratories , is in Sherwood, OR. They also do tissue testing to see what the nutrient levels are inside the plant. This is a more accurate indication of what nutrients need to be applied.
A general recommendation is: all soils are short on nitrogen; shallow rooted plants such as lawns and flowers need extra phosphorus and potassium; iron and sulfur are often deficient, especially around acid loving plants. Sometimes sandy soils need micronutrients, but rarely clay soils. Usually, the soil contains enough of the other nutrients, although some may be deficient in certain parts of the country. Boron is sometimes deficient in the Pacific Northwest. Trace minerals are more likely to be available when soil is more acid.
Many fertilizers are available to supply additional nutrients. The three numbers on packages are the percentage of Nitrogen (N), Phosphorus (P) and Potassium (K). The exact numbers are not as important as the proportions. A 5-5-5 fertilizer works the same as a 15-15-15, except that it takes three times as much 5-5-5 to provide the same nutrients. The optimum fertilizer for lawns has an N-P-K ratio of 3-1-2, such as a 15-5-10. Fertilizers with similar ratios are interchangeable, except that some fertilizers, such as rose food, might contain extra calcium carbonate to raise pH and some fertilizers, such as rhododendron-azalea food, might contain extra sulfur to lower pH.
Fertilizers come in granular form, water soluble form and liquid form. Granular forms release their nutrients in a few weeks. Some granular forms have a coating which make the nutrients release slowly over three months up to nine months for Osmocote. Granular fertilizers can be spread by hand but a fertilizer spreader will apply it more evenly. Wheel-driven drop spreaders are the most accurate but the wheel tracks have to be overlapped just the right amount to avoid gaps or double applications. Wheel-driven broadcast spreaders usually give the most uniform results. The fertilizer tapers off at the edges so there is not a sharp line if the spreader is not overlapped by the right amount. Broadcast spreaders should throw the fertilizer about as far as the last wheel track to get an even application.
Water soluble forms and liquid forms release their nutrients immediately. They need to be reapplied every week or two. They can be mixed with water and applied with a watering can or there are hose end sprayers such as Ortho Dial'N'Spray that mix water with the liquid fertilizer as it is applied. There are also hose end sprayers that are made for water soluble fertilizers. These diluted fertilizers can also be applied directly onto the leaves which will cause the leaves to turn greener in a day or two but this foliar application does not encourage much growth like soil applications do.
Some fertilizers only supply one nutrient. Many supply N, P and K only. A few fertilizers include all of the macronutrients and micronutrients. Some lawn fertilizers contain N and K but not P because if phosphorus fertilizer is washed into streams, it can cause deadly algae blooms. They still work well because there is usually plenty of phosphorus still in the soil from previous fertilizer applications.
The label on the package will tell which nutrients are included as well as the sources of the nutrients. The nutrients are identical whether they come from organic or synthetic sources, but the source will affect how fast the nutrients are available to plants. Ammonium sulfate and water soluble fertilizers release their nitrogen quickly and might burn plants if too much is applied at one time. Blood meal releases its nitrogen over a period of months. Organic fertilizers and specially treated synthetic fertilizers release slowly so they last longer and won't burn. Since phosphorus is bound up and made unavailable in a few weeks in acid soil, bone meal is much better since it releases its phosphorus over a period of several months. Deeper rooted trees and shrubs can be fertilized once a year, but shallow rooted plants, such as grass and flowers, will need regular fertilizing throughout the growing season.
Water can move nitrogen several inches in the soil. Nitrogen that is applied in the fall might be carried too deep into the soil by winter rains. February or March is the best time to feed trees and shrubs. Phosphorus and potassium hardly move in the soil. To get them down to tree roots, either punch a hole in the soil with a bar and pour in a tablespoon of granular fertilizer or use a root feeder to inject water soluble fertilizer a foot deep every two feet in rings from the trunk to beyond the length of the branches. The feeder roots are found throughout the area under and around a tree, not just at the drip line.
Home Page
Site Map
Improving Soil
Preferred Soil pH
Planting in Clay Soil